What Is an Open Reading Frame in Genetics
Introduction
Small-scale Open Reading Frames (small ORFs/sORFs/smORFs) are of import sources of putative peptides previously dismissed as beingness non-functional or junk DNA, every bit determined by early cistron prediction methods. In fact, smORFs of <100 codons are possible coding sequences but sufficiently small to occur very frequently and randomly in genomes; thus, the detection of their coding potential and functional assessment is similar to a walk in the dark. Furthermore, while dozens of smORF peptides have been recently described every bit essential players in biological processes, many are reported to exist potential not-functional products of junk DNA nether pervasive translation, leading to the question: from what perspective is this lack of function assessed? In this context, it was recently suggested that non-functional smORF peptides might play a major role during de novo poly peptide coding factor birth, but the evolutionary mechanism is yet unclear. Thus, the role of pervasive translation of smORFs in molecular development remains puzzling. Here, we present interesting questions for fence and further investigation almost the perspective of non-functional smORF peptides as underappreciated hotspots of molecular evolution in eukaryotes.
Small Open up Reading Frames: A Subtopic in the Discussion of Junk DNA Function
With respect to the evolution of molecular function, role of the DNA elements accumulate mutations past genetic drift; thus, the evolution of these elements is not-adaptive and neutral (Ohta, 2002). In some cases, the amount of neutrally evolving elements in junk Deoxyribonucleic acid are coordinating to the items on a carte du jour bachelor to natural option (Knibbe et al., 2007; Faulkner and Carninci, 2009; Lynch et al., 2011). Interestingly, it was reported by the ENCODE consortium (the Encyclopedia of DNA Elements) that almost of the human junk Deoxyribonucleic acid exhibits some blazon of biochemical activity (ENCODE Project Consortium, 2012), but lacking adaptive relevance and selective pressure (Doolittle, 2013; Graur et al., 2013). Importantly, junk DNA represents 75–ninety% of the human genome (Graur, 2017).
Office of the junk Deoxyribonucleic acid carte du jour is composed of neutrally evolving smORF peptides. For instance, thousands of not-coding RNAs are generated past the extensive transcription coverage on junk Deoxyribonucleic acid (ENCODE Project Consortium, 2007). Increasing testify shows that thousands of smORFs undergo pervasive translation in transcripts annotated as non-coding or in untranslated regions (UTR) of mRNAs (e.one thousand., Aspden et al., 2014; Ingolia et al., 2014). Interestingly, non-coding RNAs and ORFs lacking homologs were reported to be candidates for de novo evolution of protein coding genes (Tautz and Domazet-Lošo, 2011). Moreover, it was recently suggested that neutrally evolving smORF peptides might play a major office in this process (Ruiz-Orera et al., 2018), only the evolutionary mechanism remains to be determined (Ruiz-Orera et al., 2018; Singh and Wurtele, 2020). In this context, 2 previously proposed concepts used to talk over molecular role development are at the cadre of the junk Dna fence: "causal roles" and "selected effects" (Doolittle and Brunet, 2017), which will be discussed hither in the context of smORFs and protein coding factor nativity.
The "causal role" describes the activity performed by a neutrally evolving element by risk. For instance, a hypothetical genomic sequence generated by a random nucleotide mutation to resemble a TATA box may be recognized and bound past transcription factors but does not trigger gene transcription (Griffiths, 2009; Graur et al., 2013). In other words, "causal roles" are non-adaptive phenotypes, their emergence is random, and they tend to quickly disappear during evolution. On the other mitt, "selected furnishings" describe the acquisition of adaptive phenotypes based on natural option (Graur et al., 2013), such as canonical TATA boxes or ORFs that are translated into important proteins. In other words, "selected effects" are functionally relevant for cells.
Importantly, while natural selection drives adaptive development (selected furnishings), it is widely accepted that genetic migrate drives junk DNA evolution, every bit well every bit the synonymous modifications in coding Deoxyribonucleic acid sequences (CDS) and mutations in UTRs of mRNAs (Ridley, 2004).
Give-and-take
Applying the aforementioned evidence and concepts, nosotros hash out here a possible eukaryotic mechanism by which neutrally evolving smORFs advance proteome evolution and the evolutionary significance of smORFs.
Firstly, part of the roles performed by neutrally evolving smORF peptides possibly transit from "causal roles" to "selected effects" under environmental pressure level, thereby exposing their neutral phenotypes to natural selection and triggering the evolution of new coding genes. Thus, when neutral smORF peptides are selected, they are no longer neutral (Ruiz-Orera et al., 2018). In other words, neutral smORF peptides may be special entrees on the junk Deoxyribonucleic acid menu that are bachelor for natural selection (Figure 1A).
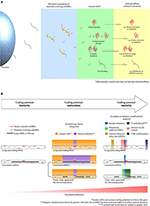
Figure 1. Phenotype selection and coding potential maturation of smORF transcripts. (A) Transition of smORF peptides from "causal roles" to "selected effects" after pervasive translation events. Pervasive translation of neutrally evolving smORFs possibly advances proteome evolution by exposing neutral phenotypes to natural selection nether ecology force per unit area. (B) Scheme for coding potential maturation, a hypothetical machinery that increase the translation efficiency of a mRNA afterward a smORF has been selected for (selected effect) in a transcript with suboptimal coding features. On the left, coding potential immaturity; in the middle, coding potential maturation; on the correct, coding potential maturity. During the coding potential immaturity phase, newly selected smORFs are observed in transcripts with suboptimal coding features, either in long not-coding RNAs or as alternative smORFs in canonical mRNAs. Although canonical mRNAs exhibit optimal coding features, alternative smORFs are usually secondarily or pervasively translated; thus, some culling smORFs may reside in suboptimal coding regions. During the coding potential maturation phase, natural pick and genetic drift may act in different parts of a transcript. While natural option acts by fixing the selected parts, genetic drift acts by changing the non-coding parts of a transcript, as postulated by the nearly neutral theory (Ohta, 2002). Natural selection promotes fine-tuned adjustments to the selected phenotypes, such every bit synonymous mutations and CDS modifications. Genetic drift tin can establish adaptive mutations in a transcript by evolving sequences that potentially increase smORF translation, such as the Kozak consensus, regulatory upstream ORFs, internal ribosome entry sites (IRES) and increases in GC content. Additionally, other adaptive modifications not straight related to sequence mutations in transcripts might increase smORF expression, such as the 5′ cap, iii′ poly(A) tail, cis-regulatory elements in the genome and, in the case of alternative smORFs, independent gene unit generation by retrotransposition. Importantly, the acquisition of optimal coding features might be favored after the smORF has been selected for, considering modifications driven by genetic migrate could be fixed by natural selection if they improve the translation efficiency of the newly selected smORF. Before the smORF has been selected for, eventual optimal coding features caused could rapidly disappear during genetic migrate evolution without fixation. Alternatively, mutations evolved by genetic drift tin can silence the gene. Finally, smORFs accomplish the coding potential maturity phase when optimal coding features are caused and translation efficiency increases. Consequently, the translation rate of smORF peptides is largely increased upon completion of the described procedure, contributing to the establishment of molecular innovations and protein coding gene birth.
Upon smORFs being selected for, they probably incorporate low adaptive relevance due to their non-coding transcript characteristics, such as depression translation rate, lack of iii′-terminal processing and other suboptimal coding features (non-coding RNA features are reviewed in Quinn and Chang, 2016). This hypothesis is based on the fact that hundreds of smORFs are described as highly conserved but display low expression, low translation efficiency and are observed in transcripts with non-coding characteristics (Cabili et al., 2011; Aspden et al., 2014; Bazzini et al., 2014). Notwithstanding, the nearly neutral theory (Ohta, 2002) suggests that non-coding parts of stock-still smORF transcripts are modified by random genetic drift, in some cases, producing small advantageous (or disadvantageous) adaptive furnishings throughout evolution; thus, nosotros propose that, at a certain point, these modifications refine and elevate the coding potential of smORF transcripts and consequently enhance the adaptive relevance of their peptides, equally seen in a big number of important smORF peptides recently discovered (due east.chiliad., Magny et al., 2013; Anderson et al., 2015; Lauressergues et al., 2015; Nelson et al., 2016; Pengpeng et al., 2017; Kim et al., 2018; Polycarpou-Schwarz et al., 2018; Chugunova et al., 2019; Tobias-Santos et al., 2019; Pang et al., 2020; Vassallo et al., 2020). Importantly, the acquisition of several optimal coding features might be favored after the smORF has been selected for, considering modifications driven by genetic drift could be stock-still by natural selection if they ameliorate the translation efficiency of the newly selected smORF. Before the smORF has been selected for, eventual optimal coding features acquired in the nucleotide sequence could rapidly disappear during genetic migrate evolution without fixation. Alternatively, nucleotide changes may negatively affect the coding potential and silence a cistron. Optimal coding features include structural stabilization, emergence of Kozak consensus, internal ribosome entry sites (IRES), coverage by enhancers and, in some cases, the elongation of coding smORFs to overstate the CDSs (coding Dna sequences) (Figure 1B). Recently, Couso and Patraquim (2017) proposed that at least a portion of functional smORFs are potential de novo precursors of large CDSs via a stop codon mutation pattern called "CDS elongation."
Because the supposition that the action of evolution is gradual, we propose that the aforementioned process be chosen "coding potential maturation" (Effigy 1B). For example, smORF translation is widely reported in transcripts with long not-coding RNA (lncRNA) characteristics (Crappé et al., 2013; Ingolia et al., 2014; Ji et al., 2015; Mackowiak et al., 2015; Li et al., 2018; Lu et al., 2019). These lncRNAs showroom smORF conservation in divergent species, hinting at natural option fixation and indicating coding immaturity.
Another potential pathway of coding cistron generation occurs via alternative smORFs in UTRs or overlapping the reference CDS of approved mRNAs. In this scenario, alternative smORFs undergo pervasive translation or the act of translation itself is important for cis-regulatory purposes (Vanderperre et al., 2013; Wu et al., 2020). If the "causal roles" performed by neutrally evolving smORF peptides go "selected furnishings," the alternative smORFs would generate independent gene units by retrotransposition, or they would exist fixed as culling smORFs in the original transcripts (Figure 1B). Hence, during retrotransposition events, at least a portion of the transcripts investigated on the footing of pseudogenization may, in fact, represent the maturation of new coding genes, equally suggested by a written report that pseudogenes can exist translated into highly conserved smORF peptides (Ji et al., 2015).
smORFs might be sequence reservoirs potentially activated during the evolution of new phenotypic variations, especially during speciation. Importantly, speciation events have been associated with the evolution of new molecular phenotypes and new relationships with the surround (Bao et al., 2018). Thus, the amount of junk Dna and lncRNAs in cells deserves investigation not only as a random accumulation of sequences and translational noise just also equally a repository of substrates to advance the development of new coding genes. Interestingly, polyploidization, or whole genome duplication (WGD) events, have been correlated with an increase in the adaptive potential of cells and organisms exposed to stressful atmospheric condition (Van De Peer et al., 2017). Unfortunately, thus far, studies of WGD take neglected the function and retention of smORFs during evolution, probably due to methodological difficulties in smORF identification.
However, the sequencing of several genomes based on comparative approaches has recently opened new avenues for smORF inquiry. For example, contempo evolutionary studies performed past our group on the smORFs in the mille-pattes/tarsalless/polished rice (mlpt) gene, the virtually well-known smORF-containing factor in insects (Savard et al., 2006; Kondo et al., 2007; Pueyo and Couso, 2008, 2011; Cao et al., 2017; Ray et al., 2019), showed that a new ~80 amino acid smORF (smHemiptera) appeared during Hemiptera evolution (Tobias-Santos et al., 2019). Thus, this smORF in the polycistronic mlpt mRNA has been conserved for over 250 million years in the grouping, and it is not present in the genomes of other insect orders. We wait that new comparative analyses of genomes in the future will yield boosted examples of order-specific smORFs, which might constitute an underappreciated reservoir of new genes and evolutionary innovations.
In summary, the study of smORFs has been considerably increasing during the concluding 5 years because of recent discoveries of important smORF peptides. Accordingly, the advent of ribosome profiling has allowed the discovery of many neutrally evolving and potentially non-functional smORFs undergoing pervasive translation, whose significance remains to exist adamant (Crappé et al., 2013; Aspden et al., 2014; Bazzini et al., 2014; Olexiouk et al., 2016). In this context, the intriguing question is posed: why would cells spend free energy on transcription and translation of neutral and non-functional elements? There is probably more than 1 answer; yet, because the subjects discussed in this paper, we propose the post-obit perspective: what if the pervasive translation of neutrally evolving smORF peptides composes an elegant mechanism to advance proteome evolution, especially during speciation events? If it does, then non-functional smORF peptides brandish an of import function in an evolutionary sense. Based on this discussion, we propose that the concept of functionality exist revised in the context of smORFs.
Author Contributions
DG-A and RN contributed equally to the writing of this manuscript. RN contributed to funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
RN was supported by CNPq (307952/2017-7 and 431354/2016-2) and FAPERJ (East-26/210-150/2016, E-26/203.298/2016, E-26/202.605/2019, and East-26/211.169/2019). DG-A was a master's student of PPG-PRODBIO-UFRJ/Macaé (CAPES scholarship).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Anderson, D. Thou., Anderson, Thou. M., Chang, C. L., Makarewich, C. A., Nelson, B. R., McAnally, J. R., et al. (2015). A micropeptide encoded past a putative long noncoding RNA regulates muscle performance. Cell 160, 595–606. doi: 10.1016/j.cell.2015.01.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Aspden, J. 50., Eyre-Walker, Y. C., Phillips, R. J., Amin, U., Mumtaz, M. A. S., Brocard, M., et al. (2014). Extensive translation of small open reading frames revealed past poly-ribo-seq. eLife 3:e03528. doi: 10.7554/eLife.03528
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bao, R., Dia, S. E., Issa, H. A., Alhusein, D., and Friedrich, Thousand. (2018). Comparative evidence of an infrequent touch of gene duplication on the developmental development of Drosophila and the higher Diptera. Front. Ecol. Evol. half-dozen:63. doi: 10.3389/fevo.2018.00063
CrossRef Full Text | Google Scholar
Bazzini, A. A., Johnstone, T. G, Christiano, R., Mackowiak, Due south. D., Obermayer, B., Fleming, East. S., et al. (2014). Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 33, 981–993. doi: 10.1002/embj.201488411
PubMed Abstract | CrossRef Full Text | Google Scholar
Cabili, M., Trapnell, C., Goff, L., Koziol, Grand., Tazon-Vega, B., Regev, A., et al. (2011). Integrative notation of human big intergenic noncoding RNAs reveals global backdrop and specific subclasses. Genes Dev. 25, 1915–1927. doi: 10.1101/gad.17446611
PubMed Abstract | CrossRef Full Text | Google Scholar
Cao, M., Gong, Y., Hu, X., Zhu, M., Liang, Z. C., Huang, L., et al. (2017). Identification of tarsal-less peptides from the silkworm Bombyx mori. Appl. Microbiol. Biotechnol. 102, 1809–1822. doi: x.1007/s00253-017-8708-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Chugunova, A., Loseva, E., Mazin, P., Mitina, A., Navalayeu, T., Bilan, D., et al. (2019). LINC00116 codes for a mitochondrial peptide linking respiration and lipid metabolism. Proc. Natl. Acad. Sci. U.Southward.A. 116, 4940–4945. doi: 10.1073/pnas.1809105116
PubMed Abstract | CrossRef Total Text | Google Scholar
Crappé, J., Criekinge, W. Five., Trooskens, 1000., Hayakawa, E., Luyten, West., Baggerman, Thousand., et al. (2013). Combining in silico prediction and ribosome profiling in a genome-wide search for novel putatively coding sORFs. BMC Genomics fourteen:648. doi: 10.1186/1471-2164-14-648
PubMed Abstruse | CrossRef Full Text | Google Scholar
Doolittle, Westward. F. (2013). Is junk Deoxyribonucleic acid bunk? A critique of ENCODE. Proc. Natl. Acad. Sci. U.S.A. 110, 5294–5300. doi: 10.1073/pnas.1221376110
CrossRef Full Text | Google Scholar
ENCODE Projection Consortium (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816. doi: x.1038/nature05874
CrossRef Total Text | Google Scholar
ENCODE Project Consortium (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. doi: x.1038/nature11247
CrossRef Full Text | Google Scholar
Graur, D., Zheng, Y., Cost, N., Azevedo, R. B. R., Zufall, R. A., Elhaik, Eastward., et al. (2013). On the immortality of telly sets: "Role" in the human genome according to the development-costless gospel of ENCODE. Genome Biol. Evol. 5, 578–590. doi: 10.1093/gbe/evt028
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ingolia, N. T., Brar, Thousand. A., Stern-Ginossar, North., Harris, Thou. S., Talhouarne, G. J. South., Jackson, Due south. East., et al. (2014). Ribosome profiling reveals pervasive translation outside of annotated poly peptide-coding genes. Cell Rep. 8, 1365–1379. doi: 10.1016/j.celrep.2014.07.045
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ji, Z., Song, R., Regev, A., and Struhl, 1000. (2015). Many lncRNAs, 5'UTRs, and pseudogenes are translated and some are likely to express functional proteins. eLife 4:e08890. doi: 10.7554/eLife.08890
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, M. H., Son, J. M., Benayoun, B. A., and Lee, C. (2018). The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 28, 516.e7–524.e7. doi: ten.1016/j.cmet.2018.06.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Knibbe, C., Coulon, A., Mazet, O., Fayard, J. M., and Beslon, Yard. (2007). A long-term evolutionary force per unit area on the corporeality of noncoding Deoxyribonucleic acid. Mol. Biol. Evol. 24, 2344–2353. doi: 10.1093/molbev/msm165
PubMed Abstract | CrossRef Full Text | Google Scholar
Kondo, T., Hashimoto, Y., Kato, Thou., Inagaki, Southward., Hayashi, Due south., and Kageyama, Y. (2007). Small-scale peptide regulators of actin-based prison cell morphogenesis encoded past a polycistronic mRNA. Nat. Prison cell Biol. 9, 660–665. doi: 10.1038/ncb1595
PubMed Abstract | CrossRef Total Text | Google Scholar
Lauressergues, D., Couzigou, J. Thou., Clemente, H. S., Martinez, Y., Dunand, C., Bécard, Thousand., et al. (2015). Primary transcripts of microRNAs encode regulatory peptides. Nature 520, 90–93. doi: 10.1038/nature14346
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, H., Xiao, L., Zhang, L., Wu, J., Wei, B., Sun, Northward., et al. (2018). FSPP: A tool for genome-wide prediction of smORF-encoded peptides and their functions. Front end. Genet. ix:96. doi: 10.3389/fgene.2018.00096
PubMed Abstruse | CrossRef Total Text | Google Scholar
Lu, S., Zhang, J., Lian, X., Sun, L., Meng, G., Chen, Y., et al. (2019). A hidden homo proteome encoded by 'non-coding' genes. Nucleic Acids Res. 47, 8111–8125. doi: 10.1093/nar/gkz646
PubMed Abstract | CrossRef Full Text | Google Scholar
Lynch, Chiliad., Bobay, L. G., Catania, F., Gout, J. F., and Rho, M. (2011). The repatterning of eukaryotic genomes past random genetic drift. Annu. Rev. Genomics Hum. Genet. 12, 347–366. doi: 10.1146/annurev-genom-082410-101412
PubMed Abstract | CrossRef Full Text | Google Scholar
Mackowiak, Due south. D., Zauber, H., Bielow, C., Thiel, D., Kutz, M., Calviello, Fifty., et al. (2015). Extensive identification and analysis of conserved minor ORFs in animals. Genome Biol. 16:179. doi: x.1186/s13059-015-0742-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Magny, E. G., Pueyo, J. I., Pearl, F. Chiliad. G., Cespedes, M. A., Niven, J. E., Bishop, S. A., et al. (2013). Conserved regulation of cardiac calcium uptake past peptides encoded in small open reading frames. Science 341, 1116–1120. doi: 10.1126/science.1238802
PubMed Abstract | CrossRef Full Text | Google Scholar
Nelson, B. R., Makarewich, C. A., Anderson, D. Yard., Winders, B. R., Troupes, C. D., Wu, F., et al. (2016). A peptide encoded past a transcript annotated as long noncoding RNA enhances SERCA action in muscle. Science 351, 271–275. doi: 10.1126/science.aad4076
PubMed Abstract | CrossRef Total Text | Google Scholar
Olexiouk, Five., Crappé, J., Verbruggen, Southward., Verhegen, G., Martens, L., and Menschaert, Thou. (2016). sORFs.org: a repository of pocket-size ORFs identified past ribosome profiling. Nucleic Acids Res. 44, D324–D329. doi: 10.1093/nar/gkv1175
PubMed Abstract | CrossRef Full Text | Google Scholar
Pang, Y., Liu, Z., Han, H., Wang, B., Li, W., Mao, C., et al. (2020). Peptide SMIM30 promotes HCC development past inducing SRC/YES1 membrane anchoring and MAPK pathway activation. J. Hepatol. doi: 10.1016/j.jhep.2020.05.028. [Epub ahead of print].
PubMed Abstruse | CrossRef Full Text | Google Scholar
Pengpeng, B., Ramirez-Martinez, A., Li, H., Cannavino, J., McAnally, J. R., Shelton, J. M., et al. (2017). Control of muscle formation by the fusogenic micropeptide myomixer. Science 356, 323–327. doi: x.1126/scientific discipline.aam9361
PubMed Abstruse | CrossRef Total Text | Google Scholar
Polycarpou-Schwarz, K., Groß, M., Mestdagh, P., Schott, J., Grund, S. Due east., Hildenbrand, C., et al. (2018). The cancer-associated microprotein CASIMO1 controls cell proliferation and interacts with squalene epoxidase modulating lipid droplet germination. Oncogene 37, 4750–4768. doi: 10.1038/s41388-018-0281-five
PubMed Abstract | CrossRef Full Text | Google Scholar
Pueyo, J. I., and Couso, J. P. (2008). The xi-aminoacid long tarsal-less peptides trigger a cell betoken in Drosophila leg development. Dev. Biol. 324, 192–201. doi: 10.1016/j.ydbio.2008.08.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Pueyo, J. I., and Couso, J. P. (2011). Tarsal-less peptides control Notch signalling through the shavenbaby transcription factor. Dev. Biol. 355, 183–1936. doi: 10.1016/j.ydbio.2011.03.033
PubMed Abstract | CrossRef Total Text | Google Scholar
Ray, S., Rosenberg, M. I., Chanut-Delalande, H., Decaras, A., Schwertner, B., Toubiana, W., et al. (2019). The mlpt/Ubr3/Svb module comprises an aboriginal developmental switch for embryonic patterning. eLife 8:e39748. doi: 10.7554/eLife.39748
PubMed Abstract | CrossRef Full Text | Google Scholar
Ridley, M. (2004). Evolution, 3rd edn. Oxford: Blackwell Pub.
Google Scholar
Ruiz-Orera, J., Verdaguer-Grau, P., Villanueva-Cañas, J. Fifty., Messeguer, Ten., and Alb,à, Grand. One thousand. (2018). Translation of neutrally evolving peptides provides a basis for de novo cistron evolution. Nat. Ecol. Evol. 2, 890–896. doi: x.1038/s41559-018-0506-half-dozen
PubMed Abstract | CrossRef Full Text | Google Scholar
Savard, J., Marques-Souza, H., Aranda, K., and Tautz, D. (2006). A segmentation factor in Tribolium produces a polycistronic mRNA that codes for multiple conserved peptides. Cell 126, 559–569. doi: 10.1016/j.jail cell.2006.05.053
PubMed Abstruse | CrossRef Full Text | Google Scholar
Tobias-Santos, V., Guerra-Almeida, D., Mury, F., Ribeiro, Fifty., Berni, Chiliad., Araujo, H., et al. (2019). Multiple roles of the polycistronic gene Tarsal-Less/Mille-Pattes/Polished-Rice during embryogenesis of the kissing bug Rhodnius prolixus. Front end. Ecol. Evol. vii:379. doi: 10.3389/fevo.2019.00379
CrossRef Full Text | Google Scholar
Vanderperre, B., Lucier, J. F., Bissonnette, C., Motard, J., Tremblay, Chiliad., Vanderperre, S., et al. (2013). Direct detection of alternative open reading frames translation products in homo significantly expands the proteome. PLoS ONE eight:e70698. doi: ten.1371/periodical.pone.0070698
PubMed Abstruse | CrossRef Full Text | Google Scholar
Vassallo, A., Palazzotto, E., Renzone, 1000., Botta, L., Faddetta, T., Scaloni, A., et al. (2020). The Streptomyces coelicolor small ORF trpM stimulates growth and morphological development and exerts opposite effects on actinorhodin and calcium-dependent antibody production. Front. Microbiol. eleven:224. doi: 10.3389/fmicb.2020.00224
PubMed Abstract | CrossRef Total Text | Google Scholar
Wu, Q., Wright, M., Gogol, K. M., Bradford, W. D., Zhang, North., and Bazzini, A. (2020). Translation of modest downstream ORFs enhances translation of approved main open up reading frames. EMBO J. 39:e104763. doi: 10.15252/embj.2020104763
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fgene.2020.574737/full
0 Response to "What Is an Open Reading Frame in Genetics"
Postar um comentário